101 The Hallmarks of Cancer
The Hallmarks of Cancer are a model for distilling the vast complexity of cancer phenotypes and genotypes into a provisional set of underlying principles. They describe cancer's functional capabilities or 'what' it does to form malignant tumors, and are generalized to describe the spectrum of human cancers. They are a useful launchpad to investigate the 'how', and subsequently what we can do about it.
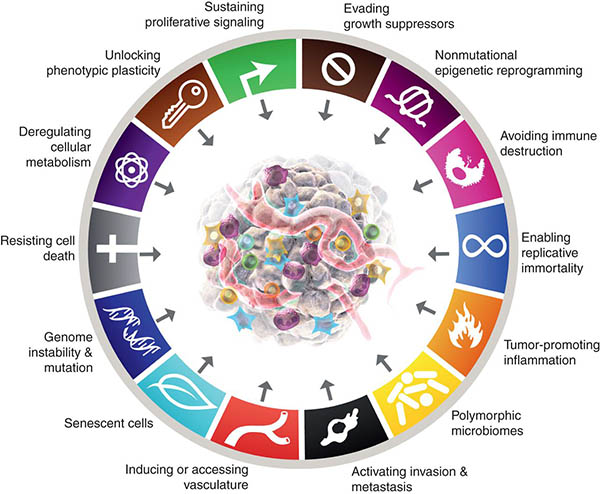
“Hallmarks of Cancer: New Dimensions” provides an update to the landmark “Hallmarks of Cancer” series. Graphic from Cancer Discovery. 1
Sustaining proliferative signalling: Self-sufficiency in growth signals
While normal cells depend on external growth signals for proliferation, cancer cells can generate most of the growth signals by themselves, greatly reducing, or eliminating, their dependence on external stimuli. A corollary to this observation was a new view of cancer as a complex tissue in which malignant cells co-opt the surrounding normal cells to provide the necessary growth signals, serving as active collaborators, rather than passive bystanders.
Evading growth suppressors: Insensitivity to growth suppressive signals
Multiple antiproliferative signals maintain the homeostasis in normal tissues, pushing cells out of the cell cycle and into a temporary quiescent state, or sending them into their terminal, post-mitotic differentiation state. Transformed cells evade these antiproliferative signals by subverting the mechanisms that control cell cycle progression—for example, by disrupting the pRb pathway, and overexpressing growth-stimulating factors such as c-myc.
Resisting cell death: Ability to evade programmed cell death
Apoptosis is a major anticancer barrier, as becoming immortal is another way through which cancer cells continue to expand in number. Further, the authors proposed that the redundancy in cell death mechanisms could be exploited for therapeutic purposes.
Enabling replicative immortality
In order for cancer to grow, malignant cells have to proliferate indefinitely. While normal cells possess a limited proliferative potential and will stop dividing at some point if cultured in vitro, cancer cells have lost that restraint mechanism, governed by telomere shortening. To become immortal, malignant cells rely on the telomerase enzyme to maintain the length of their telomeres above a critical threshold that allows them to go on dividing.
Inducing or accessing vasculature: Sustained angiogenesis
The growing tumor tissue has increased oxygen and nutrient needs and, to keep expanding, it needs to trigger the formation of new vasculature by releasing pro-angiogenic signals. At the time the authors codified this feature, it had been established that tumors go through an “angiogenic switch” that allows them to grow from microscopic to macroscopic lesions.
Activating invasion and metastasis
Metastasis is the cause of the vast majority of cancer deaths. The ability to invade, settle in, and grow in distant tissues is therefore one of the main features of cancer and relies on modifications in the cancer cell interactions with their surrounding environment through e-cadherin, integrins, and other adhesion molecules, and the production of matrix-degrading proteases.
Deregulating cellular metabolism: Reprogramming energy metabolism
While normal cells use oxygen to process glucose and produce energy, malignant cells can switch to aerobic glycolysis even in the presence of oxygen (what is known as the Warburg effect). Though this mechanism is less efficient, it is faster and originates several intermediate precursors used by cancer cells as building blocks to make proteins, DNA, and lipids to support their fast proliferation. Other cancer cells can use lactate as their main energy source.
Avoiding immune destruction
Cancer cells develop mechanisms to avoid being recognized and destroyed by the body's immune system, enabling tumor growth and spread.
Tumor-promoting inflammation
Inflammation favors multiple hallmark capabilities by providing growth, survival, and proangiogenic factors, and releasing chemicals, such as reactive oxygen species, that can cause additional mutations in the nearby cancer cells.
Genome instability and mutation
Cancers have an increased tendency for DNA mutations and other genetic changes to occur during cell division, often caused by defects in DNA repair or cell division processes. These changes, including mutations and chromosomal rearrangements, contribute to the development and progression of cancer.
Senescent Cells
Senescent cells are cells that have permanently stopped dividing (cell cycle arrest) due to damage or stress, but remain metabolically active and can release substances that may promote inflammation and potentially contribute to the development and progression of cancer.
Unlocking phenotypic plasticity and disrupted differentiation
Terminal differentiation in normal cells is associated with a permanent proliferation arrest, and increasing evidence indicates that malignant cells evade differentiation and unlock what is known as phenotypic plasticity to continue to grow. In other words, they can change their identity into something that is more inclined to proliferate. This can happen in different ways: Cells that are approaching full differentiation can de-differentiate back to a progenitor-like state; neoplastic cells originating from an undifferentiated progenitor cell can halt the differentiation process and remain in that partially differentiated, progenitor-like state; and cells that were committed to a certain differentiation phenotype can switch developmental programs, or transdifferentiate, acquiring traits that are not associated with their cell of origin. Cellular plasticity is not a “novel invention” of cancer cells, but rather a malignant twist on existing mechanisms that some normal cells can activate to repair and regenerate normal tissues.
Non-mutational epigenetic reprogramming
Global changes in the epigenetic landscape are a common feature of many cancers. Reproducing what happens during normal embryogenesis and development, cancer cells can reprogram a large number or gene-regulation networks to alter gene expression and favor the acquisition of hallmark capabilities.
Polymorphic microbiome
Our body is colonized by a vast array of microorganisms—nearly 40 trillion cells—that live in and on us. Their profound contribution to human health and disease is now appreciated. Some of these microorganisms can exert protective or deleterious effects on cancer development, progression, and response to therapy.
- Cancer Discov_ (2022) 12 (1): 31–46.
https://doi.org/10.1158/2159-8290.CD-21-1059